FDA agrees to take down Ivermectin posts in legal settlement with doctors
By Lauren Taylor (Anchor/Reporter), Evan Hummel (Producer), Jake Maslo (Video Editor)
This report was created with support from enhanced software.
Media Landscape
This story is a Media Miss by the left as only 0% of the coverage is from left leaning media. Learn moreBias Distribution
Left
Left
Untracked Bias
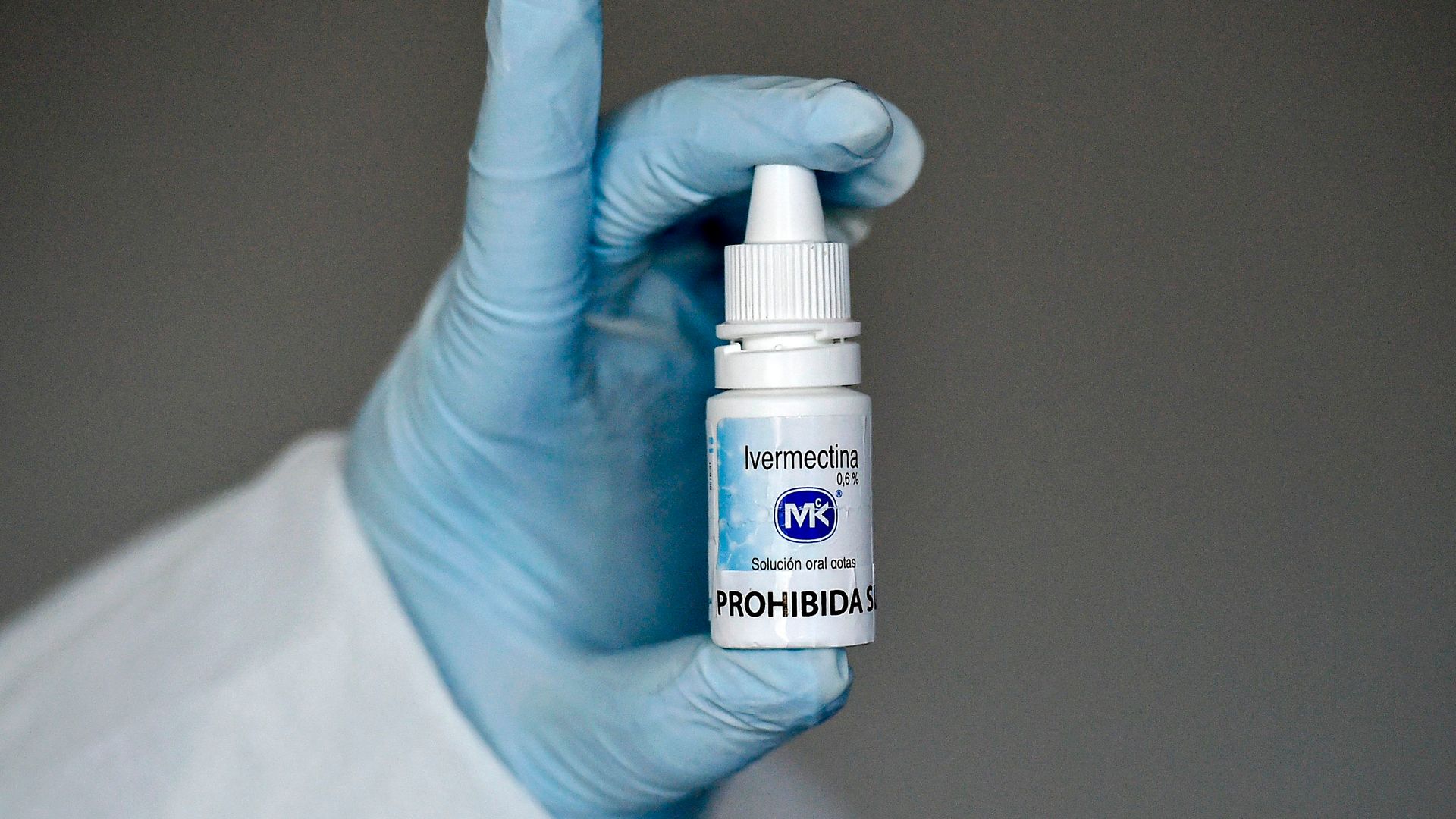
The Food and Drug Administration has reportedly agreed to remove and stop reposting several social media messages suggesting that Ivermectin — a drug used by some doctors to treat COVID-19 — is intended for animals and not humans. The move settles a lawsuit by three doctors who accused the FDA of hurting their medical practices.

Download the SAN app today to stay up-to-date with Unbiased. Straight Facts™.
Point phone camera here
The lawsuit targeted both the FDA, the U.S. Department of Health and Human Services and both agencies’ secretaries. The case was initially dismissed in June of 2022, as a judge cited the FDA’s “sovereign immunity.”
However, the U.S. Court of Appeals for the Fifth Circuit overturned the ruling on Thursday, March 21. The court said that the FDA’s role is not to provide personalized medical advice.
As a result of the latest ruling, the FDA has agreed to stop publishing a consumer update titled: “Why You Should Not Use Ivermectin to Prevent COVID-19” and remove all related social media posts within 21 days.
Ivermectin is approved for both animal and human use, and it’s used to treat some parasitic infections. However, the FDA does not recommend the drug for COVID-19 treatment, saying that there is a danger in excessive doses.
Meanwhile, Ivermectin advocates celebrated the court’s decision, and independent presidential candidate Robert F. Kennedy Jr. claimed the FDA’s stance against Ivermectin amounted to bias against “low-cost therapies.”
Unbiased news.
Directly to your inbox. Free!
Learn more about our emails. Unsubscribe anytime.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
The FDA declined to comment on Kennedy’s claims, but said again that clinical trial data does not support Ivermectin’s use to treat COVID-19.
The FDA told Newsweek that it decided to settle the lawsuit “rather than continuing to litigate over statements that are between two and nearly four years old.”
[LAUREN TAYLOR]
THE FDA AGREED TO REMOVE AND STOP REPOSTING SEVERAL SOCIAL MEDIA MESSAGES SUGGESTING IVERMECTIN, A DRUG SOME DOCTORS USED TO TREAT COVID, IS INTENDED FOR ANIMALS AND NOT HUMANS.
THE MOVE SETTLES A LAWSUIT FILED BY THREE DOCTORS WHO ACCUSED THE AGENCY OF HURTING THEIR MEDICAL PRACTICES. THE LAWSUIT TARGETED NOT ONLY THE FDA BUT THE DEPARTMENT OF HEALTH AND HUMAN SERVICES.
THE CASE WAS INITIALLY DISMISSED CITING THE FDA’S “SOVEREIGN IMMUNITY. HOWEVER, THE U.S. COURT OF APPEALS FOR THE FIFTH CIRCUIT OVERTURNED THE RULING. THE COURT SAID THE FDA’S ROLE IS NOT TO PROVIDE PERSONALIZED MEDICAL ADVICE.
AS A RESULT, THE FDA WILL NOW STOP PUBLISHING A CONSUMER UPDATE TITLED: “WHY YOU SHOULD NOT USE IVERMECTIN TO PREVENT COVID-19” AND REMOVE ALL RELATED SOCIAL MEDIA POSTS WITHIN 21 DAYS.
IVERMECTIN IS APPROVED FOR BOTH ANIMAL AND HUMAN USE, AND IS USED TO TREAT SOME PARASITIC INFECTIONS, HOWEVER, THE FDA STILL DOES NOT RECOMMEND IT FOR COVID TREATMENT, SAYING THERE IS DANGER OF EXCESSIVE DOSES.
IVERMECTIN ADVOCATES CELEBRATED THE RULING, AND INDEPENDENT PRESIDENTIAL CANDIDATE ROBERT F. KENNEDY JUNIOR CLAIMED THE FDA’S STANCE IS BIAS AGAINST “LOW-COST THERAPIES.”
THE FDA DECLINED TO COMMENT ON KENNEDY’S CLAIMS, BUT AGAIN SAID CLINICAL TRIAL DATA DOES NOT SUPPORT IVERMECTIN’S USE TO TREAT COVID.
Media Landscape
This story is a Media Miss by the left as only 0% of the coverage is from left leaning media. Learn moreBias Distribution
Left
Left
Untracked Bias
Straight to your inbox.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
MOST POPULAR
-
 Reuters
Reuters
American Airlines plane catches fire after emergency landing in Denver
Watch 1:196 hrs ago -
 Getty Images
Getty Images
Columbia announces punishments for pro-Palestine protesters
Read14 hrs ago -
 Getty Images
Getty Images
American Heart Assoc. opposes Texas SNAP regulations
Watch 5:0616 hrs ago -
 Reuters
Reuters
California takes out $3.4 billion loan after expanding health care to immigrants
Watch 1:1723 hrs ago




