FDA approves first new painkiller in decades; could help battle opioid crisis
By Craig Nigrelli (Anchor), Shea Taylor (Producer), Jack Henry (Video Editor)
The Food and Drug Administration approved a new type of pain reliever for the first time in decades. It’s a new drug that could help combat America’s opioid crisis.
Media Landscape
See how news outlets across the political spectrum are covering this story. Learn moreBias Distribution
Left
Right
Untracked Bias
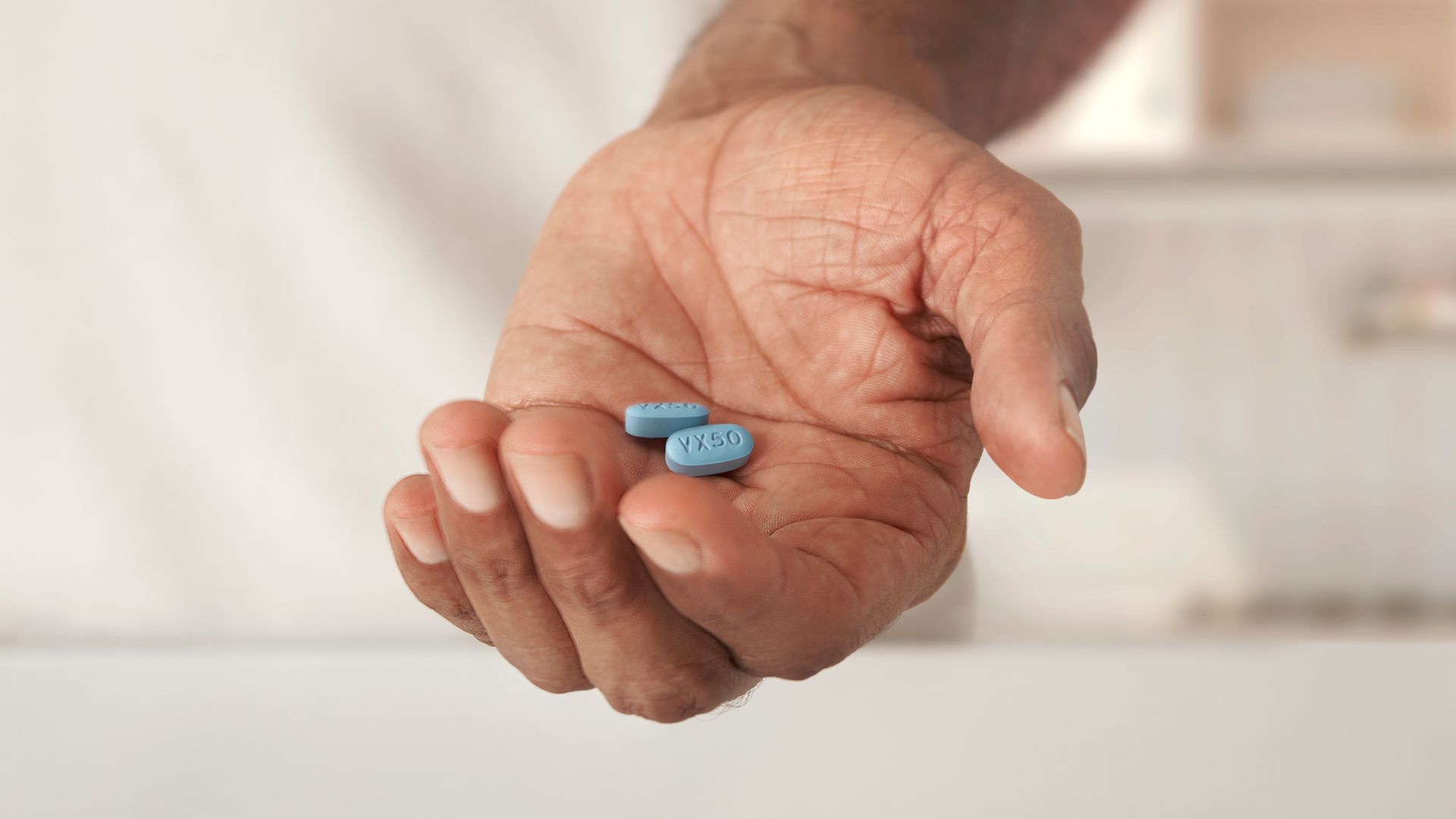
On Thursday, Jan. 30, the agency signed off on Journavx, otherwise known as suzetrigine, to treat short-term pain that often follows surgery or injuries.

Download the SAN app today to stay up-to-date with Unbiased. Straight Facts™.
Point phone camera here
The newly approved medication works as an alternative to not just opioids but also over-the-counter medications like ibuprofen and acetaminophen as well.
According to the CDC, pain medications are the most prescribed type of drug in U.S. hospitals.
The company that developed the new medication, Vertex Pharmaceuticals, said its research showed about 80 million Americans fill prescriptions each year to treat severe pain. Vertex said about half of those prescriptions are written for opioid medications.
How does suzetrigine work?
While opioid medications work by dulling the sensation of pain in the brain, suzetrigine works by preventing pain-signaling nerves around the body from firing in the first place.
On top of that, it doesn’t create the “high” some opioids are known for.
Get up to speed on the stories leading the day every weekday morning. Sign up for the newsletter today!
Learn more about our emails. Unsubscribe anytime.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
Will my insurance cover suzetrigine?
It’s not yet clear if insurance companies will cover this new drug.
Right now, Vertex has set a wholesale cost of $15.50 per 50 mg pill. That’s much more expensive than comparable opioids, which are often available as generics for $1 or less per pill.
Vertex previously said patient assistance programs would be available.
[CRAIG NIGRELLI]
THE FOOD AND DRUG ADMINISTRATION HAS APPROVED A NEW TYPE OF PAIN RELIEVER – FOR THE FIRST TIME IN DECADES – AND IT COULD HELP COMBAT AMERICA’S OPIOID CRISIS.
ON THURSDAY, THE AGENCY SIGNED OFF ON JOURNAVX – OR SUZETRIGINE – TO TREAT THE SHORT-TERM PAIN THAT OFTEN FOLLOWS SURGERY OR INJURIES, KNOWN IN THE MEDICAL WORLD AS “ACUTE PAIN.”
THE NEWLY-APPROVED MEDICATION IS AN ALTERNATIVE TO NOT JUST OPIOIDS – BUT OVER-THE-COUNTER MEDICATIONS LIKE IBUPROFEN AND ACETAMINOPHEN, AS WELL.
ACCORDING TO THE C-D-C – PAIN MEDICATIONS ARE THE MOST PRESCRIBED TYPE OF DRUG IN U-S HOSPITALS.
ACCORDING TO THE COMPANY THAT DEVELOPED THE NEW DRUG – VERTEX PHAMACEUTICALS – ABOUT 80 MILLION AMERICANS FILL PRESCRIPTIONS EACH YEAR FOR MEDICATIONS TO TREAT NEW INSTANCES OF MODERATE TO SEVERE PAIN.
VERTEX SAYS ABOUT HALF THOSE PRESCRIPTIONS ARE WRITTEN FOR OPIOID MEDICATIONS.
WHILE OPIOID MEDICATIONS WORK BY DULLING THE SENSATION OF PAIN IN THE BRAIN… SUZETRIGINE WORKS BY PREVENTING PAIN-SIGNALING NERVES AROUND THE BODY FROM FIRING IN THE FIRST PLACE.
ON TOP OF THAT – IT DOESN’T CREATE THE “HIGH” SOME OPIOIDS ARE KNOWN FOR.
IT’S NOT YET CLEAR IF INSURANCE COMPANIES WILL COVER THIS NEW DRUG.
STAY UP TO DATE AN ALL THE LATEST MEDICAL INNOVATIONS LIKE THIS BY DOWNLOADING THE STRAIGHT ARROW NEWS APP TODAY.
Media Landscape
See how news outlets across the political spectrum are covering this story. Learn moreBias Distribution
Left
Right
Untracked Bias
Straight to your inbox.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
MOST POPULAR
-
 Getty Images
Getty Images
DHS Secretary Noem tells wildfire victims to protect themselves and property
Watch 1:527 hrs ago -
 Getty Images
Getty Images
Former Social Security head claims DOGE will cause payment interruptions
Read8 hrs ago -
 Getty Images
Getty Images
Gen Z's approval rating constantly fluctuating
Watch 3:419 hrs ago -
 Getty Images
Getty Images
Trump to put tariffs on agricultural imports, tells farmers to ‘have fun’
Watch 2:0712 hrs ago




