Supreme Court to hear oral arguments in abortion pill case
By Lauren Taylor (Reporter), Zachary Hill (Video Editor)
Media Landscape
See how news outlets across the political spectrum are covering this story. Learn moreBias Distribution
Left
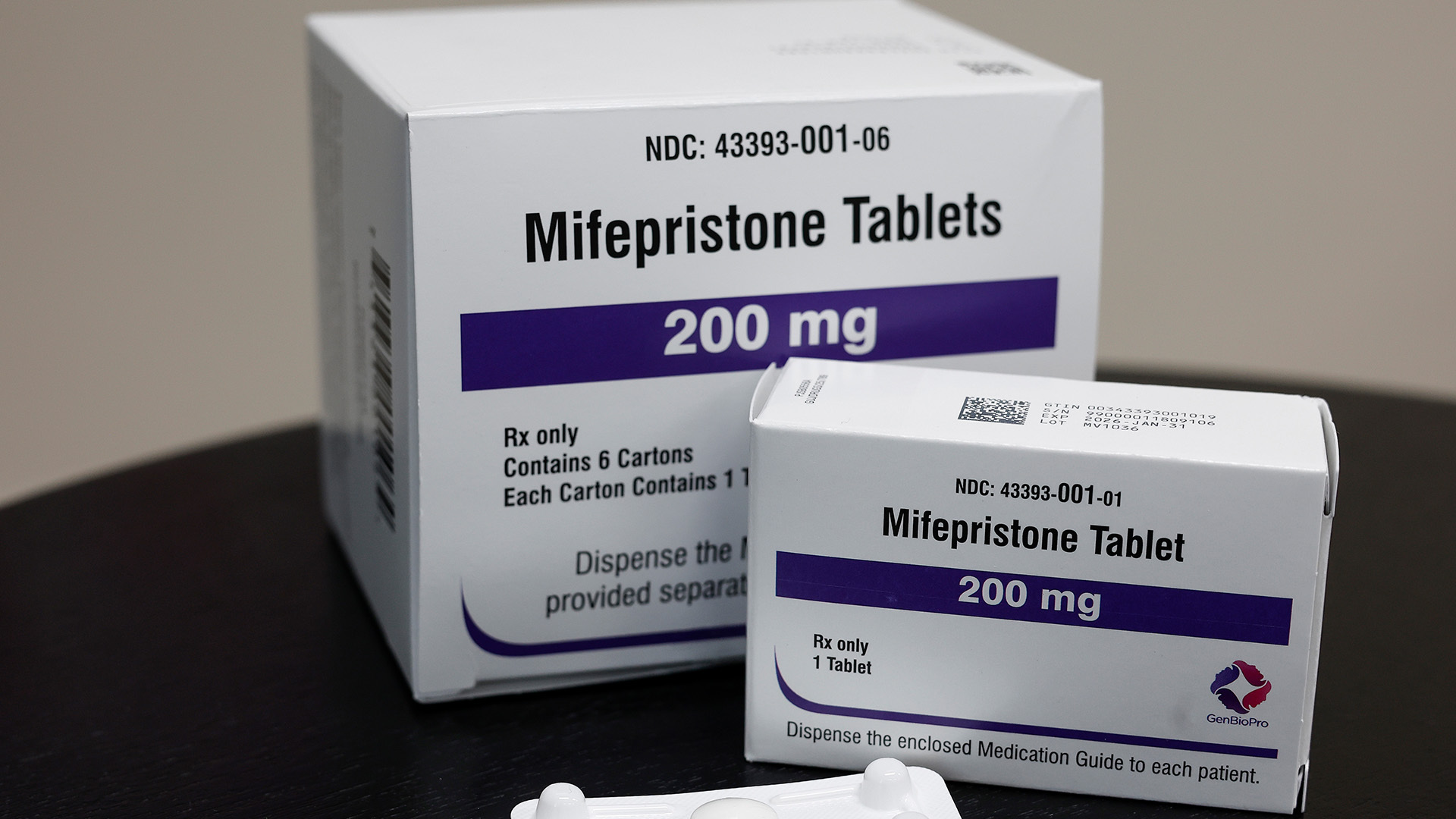
The U.S. Supreme Court is set to hear its first abortion case since the controversial Dobbs decision that overturned Roe v. Wade. The high court announced it will hear oral arguments in the case on access to abortion drug, mifepristone, in March.
Mifepristone is one of two abortion drugs used to end a pregnancy.

Download the SAN app today to stay up-to-date with Unbiased. Straight Facts™.
Point phone camera here
“It works by stopping the supply of hormones that maintains the interior of the uterus,” a Mayo Clinic drug information sheet stated. “Without these hormones, the uterus cannot support the pregnancy and the contents of the uterus are expelled.”
Medication abortions account for half of abortions in the United States, according to the CDC. Mifepristone has been used in 5 million abortion cases, and the FDA said adverse events associated with its use are low. The FDA approved mifepristone for use back in 2000.
In June 2022, the Supreme Court overturned Roe, ruling the Constitution does not confer a right to abortion. Later that year in November, a group of physicians with the Alliance for Hippocratic Medicine, which opposes abortion, brought a challenge against the FDA’s mifepristone approval.
The group argued the FDA did not adequately consider the safety of mifepristone before approving the drug.
The plaintiffs argued to reverse FDA measures implemented since 2016 that made mifepristone easier to obtain and more unsafe for patient use. Those measures included:
- Allowing the pill to be taken later in pregnancy at 10 weeks, as opposed to 7 weeks.
- Creating a generic version of the drug.
- Allowing doctors to prescribe it and distribute it by mail.
In April 2023, a U.S. District Court judge in Texas put a hold on the FDA’s 2000 approval of mifepristone. The Biden administration then appealed to the 5th Circuit Court of Appeals.
If a judge decides to substitute his preferences, his personal opinion, for that of scientists and medical professionals, what drug isn’t subject to some kind of legal challenge?
Xavier Becerra, HHS secretary
“First and foremost, when you turn upside down the entire FDA approval process, you’re not talking about just mifepristone, you’re talking about every kind of drug,” said Health and Human Services Secretary Xavier Becerra. “You’re talking about our vaccines, you’re talking about insulin, you’re talking about the new Alzheimer’s drugs that may come on. If a judge decides to substitute his preferences, his personal opinion, for that of scientists and medical professionals, what drug isn’t subject to some kind of legal challenge?”
The 5th Circuit Court of Appeals ruled in favor of the FDA’s original approval of mifepristone. However, the three-judge panel nullified the changes put in place by the FDA that made mifepristone easier to access in 2016.
The FDA argued if those restrictions are allowed to take place, it would cause widespread chaos. So, the Supreme Court then intervened and blocked that ruling, allowing mifepristone to stay on the market with no changes to how it’s prescribed while the case plays out in the lower courts.
Mifepristone’s manufacturer, Danco Laboratories, and the Department of Justice asked the Supreme Court to review the 5th Circuit Court’s ruling, which the high court agreed to in December.
Oral arguments are set for March 26 and a decision is expected to come down in June.
Unbiased news.
Directly to your inbox. Free!
Learn more about our emails. Unsubscribe anytime.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
[LAUREN TAYLOR]
THE U-S SUPREME COURT IS SET TO HEAR ITS FIRST ABORTION CASE SINCE ITS CONTROVERSIAL DOBBS DECISION OVERTURNING ROE VERSUS WADE.
THE HIGH COURT ANNOUNCED IT WILL HEAR ORAL ARGUMENTS – IN THE CASE OF ACCESS TO AN ABORTION DRUG – CALLED “MIFEPRISTONE” – ON MARCH 26TH.
MIFEPRISTONE IS ONE OF TWO ABORTION DRUGS THAT’S USED TO END A PREGNANCY…
AND ACCORDING TO THE MAYO CLINIC – “IT WORKS BY STOPPING THE SUPPLY OF HORMONES THAT MAINTAINS THE INTERIOR OF THE UTERUS. WITHOUT THESE HORMONES, THE UTERUS CANNOT SUPPORT THE PREGNANCY AND THE CONTENTS OF THE UTERUS ARE EXPELLED.”
MIFEPRISTONE HAS BEEN USED IN 5-MILLION ABORTION CASES AND THE FDA SAYS ADVERSE EVENTS ASSOCIATED WITH USE OF THE DRUG ARE LOW.
MEDICATION ABORTIONS ACOCUNT FOR HALF OF ABORTIONS IN THE UNITED STATES – ACCORDING TO THE CDC.
SO HOW DID WE GET HERE?
THE FDA APPROVED MIFEPRISTONE FOR USE IN THE YEAR 2000.
IN JUNE 2022 – THE SUPREME COURT OVERTURNED ROE, SAYING THE CONSTITUTION DOES NOT CONFER A RIGHT TO ABORTION
THEN – IN NOVEMBER 2022 – A GROUP OF PHYSICIANS with the alliance for hippocratic medicine – which OPPOSEs ABORTION – BROUGHT A CHALLENGE AGAINST THE FDA’S 2000 APPROVAL OF MIFEPRISTONE.
THE GROUP ARGUED – THE FDA DID NOT CONSIDER THE SAFETY OF MIFEPRISTONE BEFORE APPROVING THE DRUG FOR USE. THE DOCTORS ALSO ARGUED TO ROLL BACK STEPS THE FDA HAS TAKEN SINCE 2016 – THAT MADE MIFEPRISTONE EASIER TO OBTAIN AND, THEY SAY, MORE UNSAFE FOR PATIENT USE. THOSE STEPS INCLUDED – ALLOWING THE PILL TO BE TAKEN LATER IN PREGNANCY AT 10 WEEKS – AS OPPOSED TO 7 WEEKS… CREATING A GENERIC VERSION OF THE DRUG… AND ALLOWING DOCTORS TO PRESCRIBE IT AND DISTRIBUTE IT BY MAIL.
IN APRIL 2023 – A U.S. DISTRICT COURT JUDGE IN TEXAS PUT A HOLD ON THE FDA’S 2000 APPROVAL OF MIFEPRISTONE.
THE BIDEN ADMINISTRATION THEN APPEALED TO THE 5TH CIRCUIT COURT OF APPEALS.
[XAVIER BECERRA / HHS SECRETARY]
“FIRST AND FOREMOST, WHEN YOU TURN UPSIDE DOWN THE ENTIRE FDA APPROVAL PROCESS, YOU’RE NOT TALKING ABOUT JUST MIFEPRISTONE, YOU’RE TALKING ABOUT EVERY KIND OF DRUG. YOU’RE TALKING ABOUT OUR VACCINES, YOU’RE TALKING ABOUT INSULIN, YOU’RE TALKING ABOUT THE NEW ALZHEIMER’S DRUGS THAT MAY COME ON. IF A JUDGE DECIDES TO SUBSTITUTE HIS PREFERENCES, HIS PERSONAL OPINION, FOR THAT OF SCIENTISTS AND MEDICAL PROFESSIONALS, WHAT DRUG ISN’T SUBJECT TO SOME KIND OF LEGAL CHALLENGE, SO WE HAVE TO GO TO COURT. AND FOR WOMEN’S SAKE, WE HAVE TO PREVAIL IN THIS.”
[LAUREN TAYLOR]
THEN – THE 5TH CIRCUIT COURT OF APPEALS ruled THE FDA’S ORIGINAL APPROVAL OF MIFEPRISTONE IN 2000 WOULD REMAIN IN EFFECT – HOWEVER – THE 3-JUDGE-PANEL NULLIFIED THE CHANGES PUT IN PLACE BY THE FDA – THAT MADE MIFEPRISTONE EASIER TO ACCESS IN 2016.
THE ADMINISTRATION THEN ARGUED – IF THOSE RESTRICTIONS ARE ALLOWED TO TAKE PLACE – IT WOULD CAUSE WIDESPREAD CHAOS.
SO — THE SUPREME COURT THEN INTERVENED – AND BLOCKED THAT RULING – ALLOWING MIFEPRISTONE TO STAY ON THE MARKET WITH NO CHANGES TO HOW IT’S PRESCRIBED – WHILE THE CASE PLAYS OUT IN THE LOWER COURTS.
MIFEPRISTONE’S MANUFACTURER – danco LABORATORIES AND THE DEPARTMENT OF JUSTICE – ASKED THE SUPREME COURT TO REVIEW THE 5TH CIRCUIT COURT’S RULING – WHICH THE HIGH COURT AGREED TO DO IN DECEMBER.
ORAL ARGUMENTS ARE SET FOR MARCH – AND A DECISION IS LIKELY TO COME DOWN IN JUNE.
Media Landscape
See how news outlets across the political spectrum are covering this story. Learn moreBias Distribution
Left
Straight to your inbox.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
MOST POPULAR
-
 Getty Images
Getty Images
Trump preparing to sign executive order to begin closing Department of Education
Read8 hrs ago -
 Getty Images
Getty Images
Republicans must cut Medicaid to fund Trump’s agenda, budget office says
Watch 2:109 hrs ago -
 Getty Images
Getty Images
Trump to revoke legal status for 240,000 Ukrainians as deportations rise: Report
Read10 hrs ago -
 U.S. Department of Energy
U.S. Department of Energy
Alaska’s $44B natural gas pipeline project attracts foreign interest
Watch 1:3911 hrs ago




