Publisher retracts key study cited by federal judge in FDA abortion pill ruling
By Lauren Taylor (Anchor), Jake Maslo (Video Editor)
Media Landscape
This story is a Media Miss by the right as only 3% of the coverage is from right leaning media. Learn moreBias Distribution
Left
Right
Right
Untracked Bias
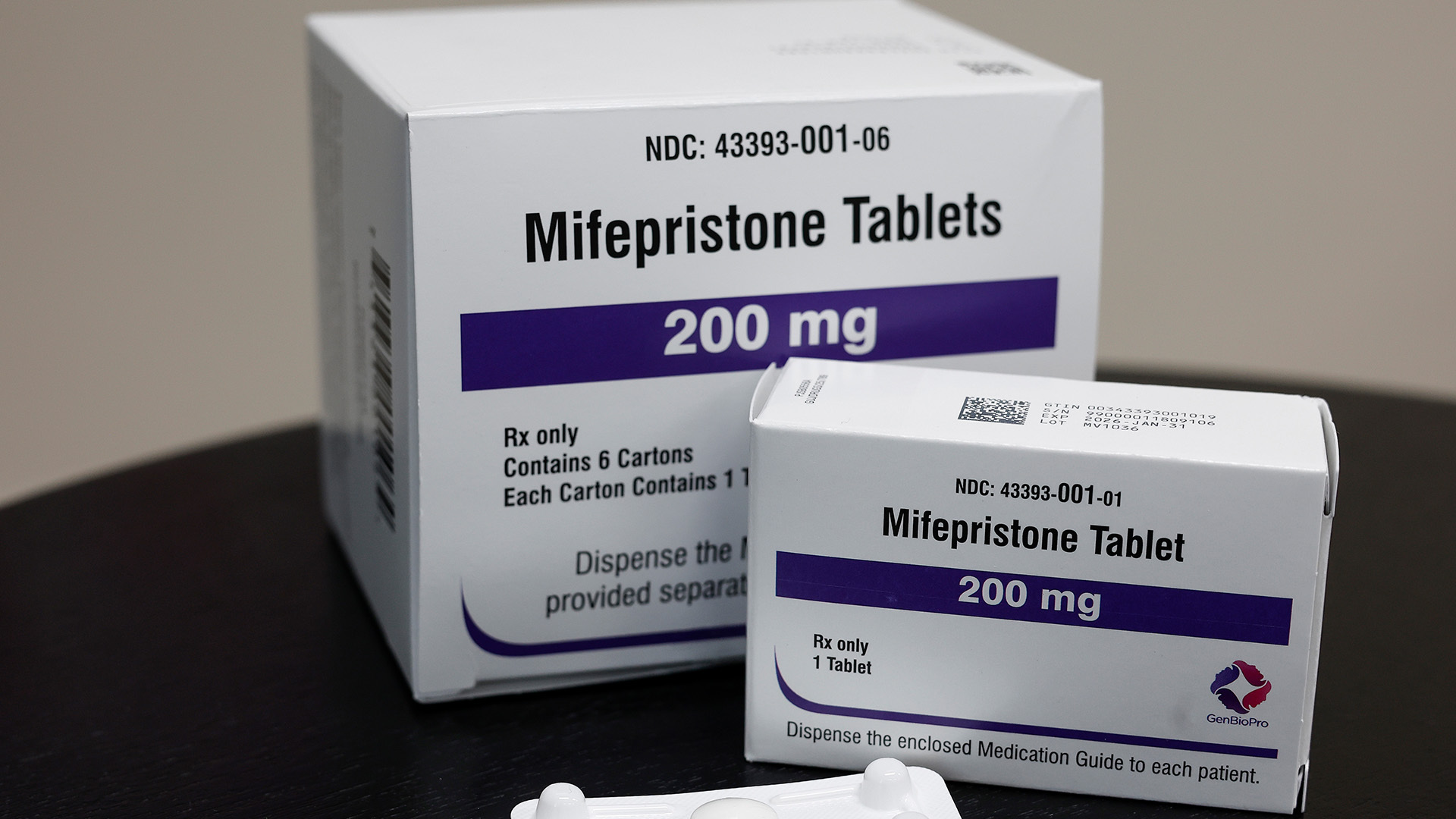
Last year, a Texas federal judge’s decision to revoke the Food and Drug Administration’s (FDA) approval of the abortion pill mifepristone ignited a nationwide controversy over abortion rights. The legal skirmish over the pill has escalated further following the retraction of a key scientific paper that played a pivotal role in the case.

Download the SAN app today to stay up-to-date with Unbiased. Straight Facts™.
Point phone camera here
In 2021, Sage Journals published a study suggesting that mifepristone significantly increases the risk of women requiring emergency room care after an abortion. This study was cited by Texas District Judge Matthew Kacsmaryk in his April ruling against the FDA’s approval of the pill. Recently, Sage announced the retraction of this study and two others by lead author James Studnicki, criticizing the research for its lack of scientific rigor and accusing the author of undeclared conflicts of interest.
Sage commissioned two independent experts for a post-publication review of the papers. The experts identified “fundamental problems,” undermining the authors’ conclusions. Studnicki, in a rebuttal shared with Wired, denounced the retractions as “unjustified” and described Sage’s action as a “baseless ideological attack.”
Sage highlighted that Studnicki and his co-authors had declared no conflicts of interest upon submitting their research. However, Studnicki serves as vice president of the Charlotte Lozier Institute, the research arm of Susan B. Anthony Pro-Life America, a prominent anti-abortion organization.
Unbiased news.
Directly to your inbox. Free!
Learn more about our emails. Unsubscribe anytime.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
Studnicki maintains that his affiliation with anti-abortion groups was “fully known and identified to Sage” at the time of submission.
The future of the Texas ruling now rests with the Supreme Court, following a federal appeals court’s decision that mifepristone should stay accessible to patients while the lawsuit against the FDA proceeds.
The Supreme Court is scheduled to hear arguments on the matter in March, marking the first abortion case since the landmark 2021 Dobbs decision, which overturned Roe v. Wade and ended federal protection for abortion rights.
[LAUREN TAYLOR]
A FEDERAL JUDGE IN TEXAS LAST YEAR REVOKED THE FOOD AND DRUG ADMINISTRATION’S APPROVAL OF THE ABORTION PILL MIFEPRISTONE — TOUCHING OFF A FIRESTORM OF DEBATE OVER ABORTION RIGHTS.
NOW, A PUBLISHED SCIENTIFIC PAPER AT THE CENTER OF THE ABORTION PILL LEGAL BATTLE HAS BEEN RETRACTED.
IN 20-21, SAGE JOURNALS PUBLISHED A STUDY PURPORTING TO SHOW MIFEPRISTONE SIGNIFICANTLY INCREASES WOMEN’S RISK OF EMERGENCY ROOM VISITS FOLLOWING AN ABORTION.
TEXAS DISTRICT JUDGE MATTHEW KACSMARYK CITED THE STUDY IN HIS APRIL RULING AGAINST THE F-D-A’S APPROVAL OF THE PILL.
THIS WEEK, SAGE ANNOUNCED IT WAS RETRACTING THE STUDY — AND TWO OTHERS — BY LEAD AUTHOR JAMES STUDNICKI. THE PUBLISHER CLAIMING THE STUDIES SHOWED A “LACK OF SCIENTIFIC RIGOR” AND ACCUSING THE AUTHOR OF UNDECLARED CONFLICTS OF INTEREST.
ACCORDING TO SAGE, TWO INDEPENDENT EXPERTS CONDUCTED A POST-PUBLICATION REVIEW OF THE THREE PAPERS AND FOUND WHAT THEY CALLED “FUNDAMENTAL PROBLEMS” THAT INVALIDATED THE AUTHORS’ CONCLUSIONS.
IN A DETAILED REBUTTAL TO SAGE PUBLISHING SHARED WITH “WIRED,” STUDNICKI CALLED THE RETRACTIONS “UNJUSTIFIED.” HE LATER CHARACTERIZED SAGE’S MOVE AS A “BASELESS IDEOLOGICAL ATTACK.”
WHEN IT COMES TO THE ALLEGATIONS OF CONFLICT OF INTEREST, SAGE SAID STUDNICKI AND THE OTHER AUTHORS DECLARED THEY HAD NOT CONFLIGHTS WHEN THEY SUBMITTED THE STUDY.
STUDNICKI IS VICE PRESIDENT OF THE CHARLOTTE LOZIER INSTITUTE, THE RESEARCH ARM OF SUSAN B. ANTHONY PRO-LIFE AMERICA, A LEADING ANTI-ABORTION GROUP.
ACCORDING TO STUDNICKI, HIS AND HIS FELLOW AUTHORS’ CONNECTION TO ANTI-ABORTION GROUPS WAS “FULLY KNOWN AND IDENTIFIED TO SAGE” WHEN THE PAPERS WERE SUBMITTED.
THE TEXAS RULING IS NOW IN THE HANDS OF THE SUPREME COURT AFTER A FEDERAL APPEALS COURT RULED MIFEPRISTONE SHOULD REMAIN AVAILABLE TO PATIENTS WHILE THE LAWSUIT AGAINST THE F-D-A CONTINUES IN THE COURTS.
JUSTICES ON THE HIGH COURT ARE SET TO HEAR ARGUMENTS OVER THE ISSUE NEXT MONTH. IT WILL BE THE FIRST ABORTION CASE THE COURT HAS RULED ON SINCE THE 20-21 DOBBS DECISION WHICH OVERTURNED ROE V WADE — EFFECTIVELY REMOVING FEDERAL PROTECTION FOR ABORTION RIGHTS.
Media Landscape
This story is a Media Miss by the right as only 3% of the coverage is from right leaning media. Learn moreBias Distribution
Left
Right
Right
Untracked Bias
Straight to your inbox.
By entering your email, you agree to the Terms & Conditions and acknowledge the Privacy Policy.
MOST POPULAR
-
 Reuters
Reuters
It’s a bird, it’s a plane, it’s the first video of Alef Aeronautics’ flying car
Watch 2:1315 hrs ago -
 Getty Images
Getty Images
Democrats in Congress receive lowest approval rating in Quinnipiac poll history
Watch 2:5916 hrs ago -
 Getty Images
Getty Images
AG Bondi reviewing Epstein documents for release, could hold client list
Watch 1:4816 hrs ago -
 Getty Images
Getty Images
Speaker Johnson won’t support DOGE stimulus checks
Watch 2:0618 hrs ago




